A synergistic effect for the well-being of the gastrointestinal system
Despite preventive measures, enteric infections in newborn calves are one of the leading causes of calf mortality. With new regulations limiting the prophylactic use of antimicrobials, there is an urgent need for alternative approaches to minimize the incidence of diarrhea in newborn calves.
Methods are needed to improve calf gut health during the pre-weaning period to minimize their susceptibility to enteric infections. Manipulating the gut microbiome is a key factor that influences gut health.
Calf gut microbiome and microbiota – definitions
The terms microbiota and microbiome refer to a heterogeneous population of microscopic organisms residing in a defined space at a given time. The microbiota includes microorganisms such as bacteria, fungi, archaea, protozoa, and viruses that live in and colonize a specific environment.
The term microbiome, on the other hand, refers to the genetic material possessed by the microbiota, specifically the totality of genes expressed by the microorganisms. The total number of genes in the microbiota is estimated to be 100 times the number of genes in the human genome. The microbial genome shares 99% of its genes with the human genome. Thus, while microbiota and microbiome are distinct concepts, they are closely related.
Changes in the microbiota lead to alterations in the microbiome, impacting the body’s homeostasis. The microbiota can be divided into bacteriota (the bacterial component), virota (the viral component), and mycota (the fungal component). Generally, when referring to microbiota, the bacterial component is emphasized due to the greater capacity of bacteria to metabolize digestive products, including the fermentation of carbohydrates and proteins, which produces fatty acids, hydrogen, carbon dioxide, ammonia, amines, phenols, and energy.
The intestinal microbiome plays a crucial role in the development, maturation, and homeostasis of the mucosal immune system, and dysbiosis in the intestinal microbiome has been linked to various enteric disorders that cause inflammation of the intestinal tissue. Maintaining normal bacterial density and composition is essential for a healthy gut.
There is a true symbiosis between the epithelial cells of the intestinal mucosa and the bacteria, facilitated by specific epithelial membrane receptors that can recognize and distinguish the microbiome. The mucosal immune system can influence changes in the microbiome, and vice versa.
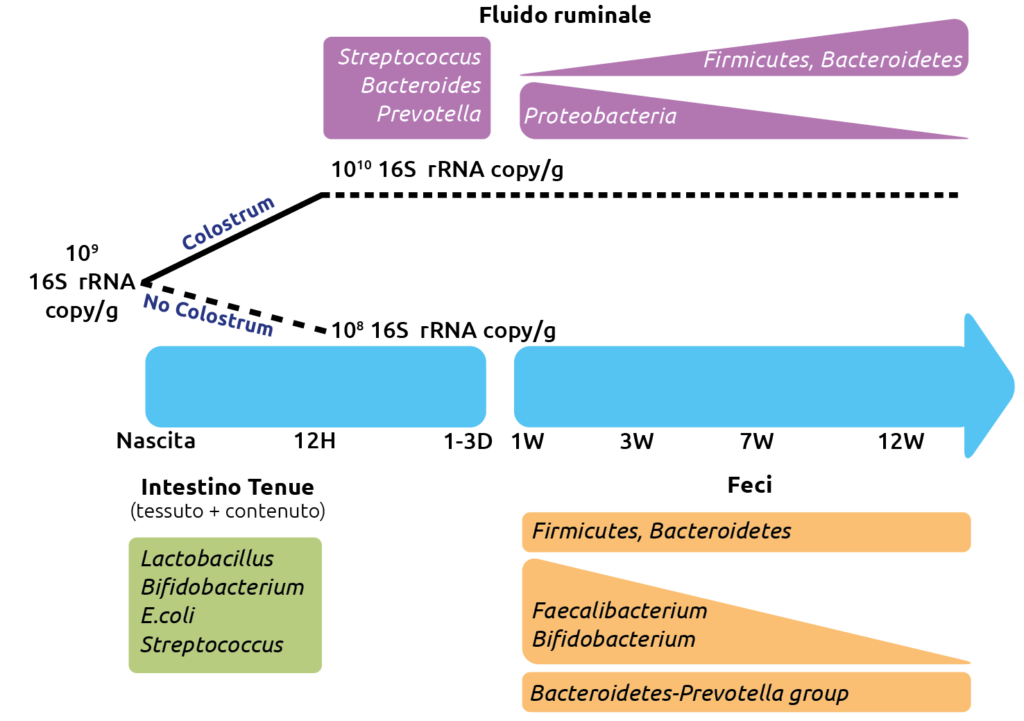
Microbioma intestinale del vitello pre-svezzato: cosa ne sappiamo?
The calf’s intestinal microbiome is already present in the fetus and, therefore, in the meconium, and it changes with age and diet. Recent studies have reported the existence of a diverse microbiota in fetuses of calves aged between 5 and 7 months, suggesting that the colonization of the bovine intestine by the so-called “pioneer” microbiota might begin as early as mid-gestation.
It has also been shown that maternal nutritional regimen and prenatal supplementation with vitamins and trace elements induce changes in the uterine microbial community, which can influence the colonization of the intestines, vagina, and ocular surface of the calves.
Finally, the calf’s intestinal microbiota changes based on the genetics of the animals and the specific section of the intestine.
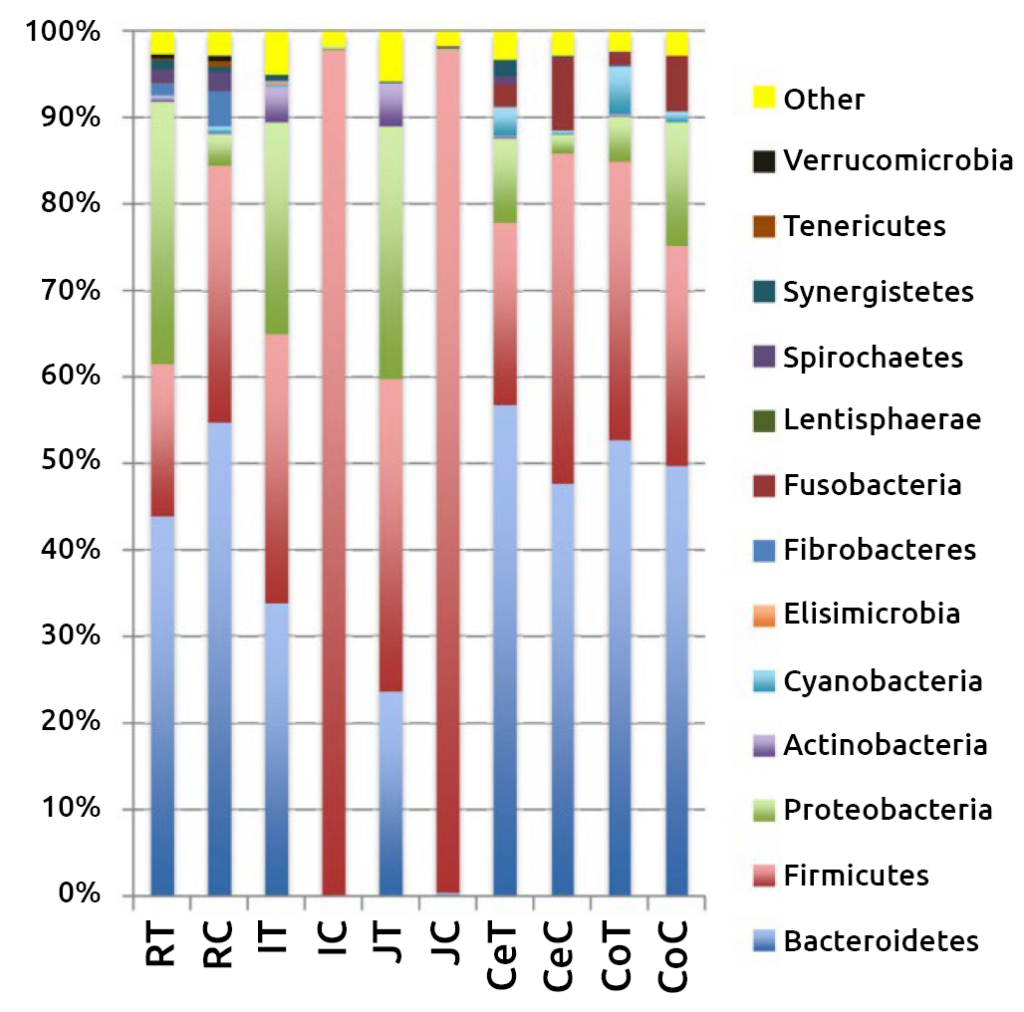
The importance of the first week
Initial colonizers (Streptococcus and Enterococcus) use the available oxygen in the intestine and create the anaerobic environment required for strictly anaerobic intestinal residents, such as Bifidobacterium and Bacteroides. The presence of Bacteroides in the gut plays a vital role in the development of immunological tolerance to the commensal microbiota, while the composition of Bifidobacterium in the gut is associated with a reduced incidence of allergies. Therefore, neonatal intestinal colonization is a critical period for the development of the gut and naïve immune system and can have long-term health effects. Early studies on bacterial colonization of the pre-ruminant gut primarily focused on pathogenic Escherichia coli in calves and described the pathogenesis of neonatal diarrhea.
Microscopic examination revealed that pathogenic E. coli preferentially adheres to and damages the epithelial lining in the ileum and large intestine, but not in the duodenum and jejunum of newborn calves. Administration of probiotic strains isolated from the calves’ intestines reduced enteric colonization by the pathogen E. coli O157:H7 in pre-weaned calves. Similarly, administration of Bifidobacterium and Lactobacillus to newborn calves during the first week of life improved weight gain and feed conversion ratio, while simultaneously reducing the incidence of diarrhea.
These effects were more pronounced in pre-weaned calves compared to weaned calves, suggesting that probiotic supplements are more effective when the calf’s intestinal microbiota is still stabilizing and less effective once the microbiome has stabilized. Administration of Lactobacillus in young calves increases the serum concentration of total immunoglobulin G (Al-Saiady J Anim Vet Adv (2010)), providing evidence of a host-microbiome interaction that can influence calf health.
More recently, supplementation of newborn calves with prebiotics (galactooligosaccharides) has been associated with an increased abundance of Lactobacillus and Bifidobacterium in the colon of 2-week-old calves (Marquez CJ, University of Illinois (2014)). However, this effect was less pronounced in 4-week-old calves, suggesting that, similar to probiotics, it may be easier to manipulate the microbiome during the initial colonization period.
Mucosal Immune System in Calves
The mucosal immune system includes physical barriers (mucosal layer, epithelium), chemical barriers (antimicrobial peptides, secretory IgA), and pattern recognition receptors (toll-like receptors (TLR), NOD-like receptors), as well as a repertoire of cellular immune defenses (innate and adaptive).
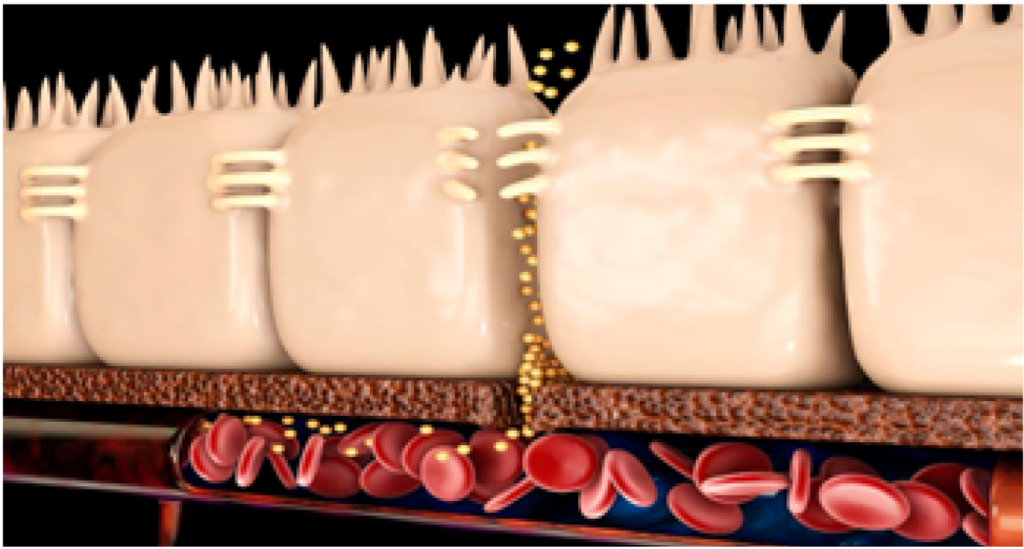
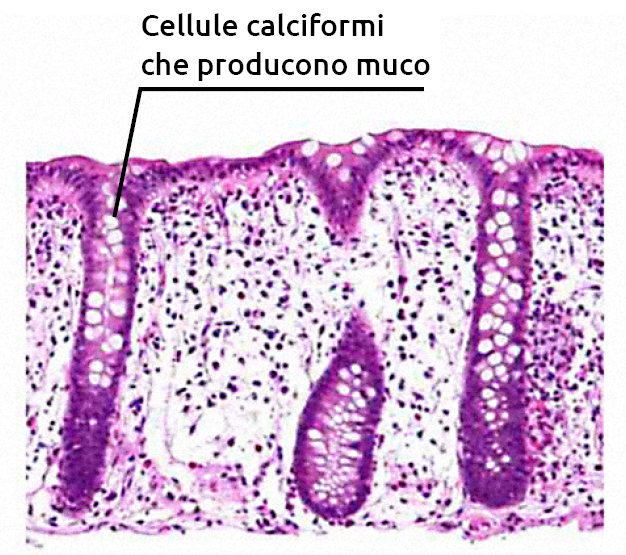
The mucosal layer, the first secretory physical barrier, contains glycosylated mucins in a network form that trap the microbiota. Secretory compounds (IgA, antimicrobial peptides) influence the growth of the microbiota on the mucosal layer. Beneath the mucosal layer, a single layer of epithelial cells, connected by an intracellular junction complex (tight junctions), regulates the movement of macromolecules across the epithelial layer. The calf’s intestinal microbiota is essential for the secretion of intestinal mucus, an important physical barrier throughout the gastrointestinal tract.
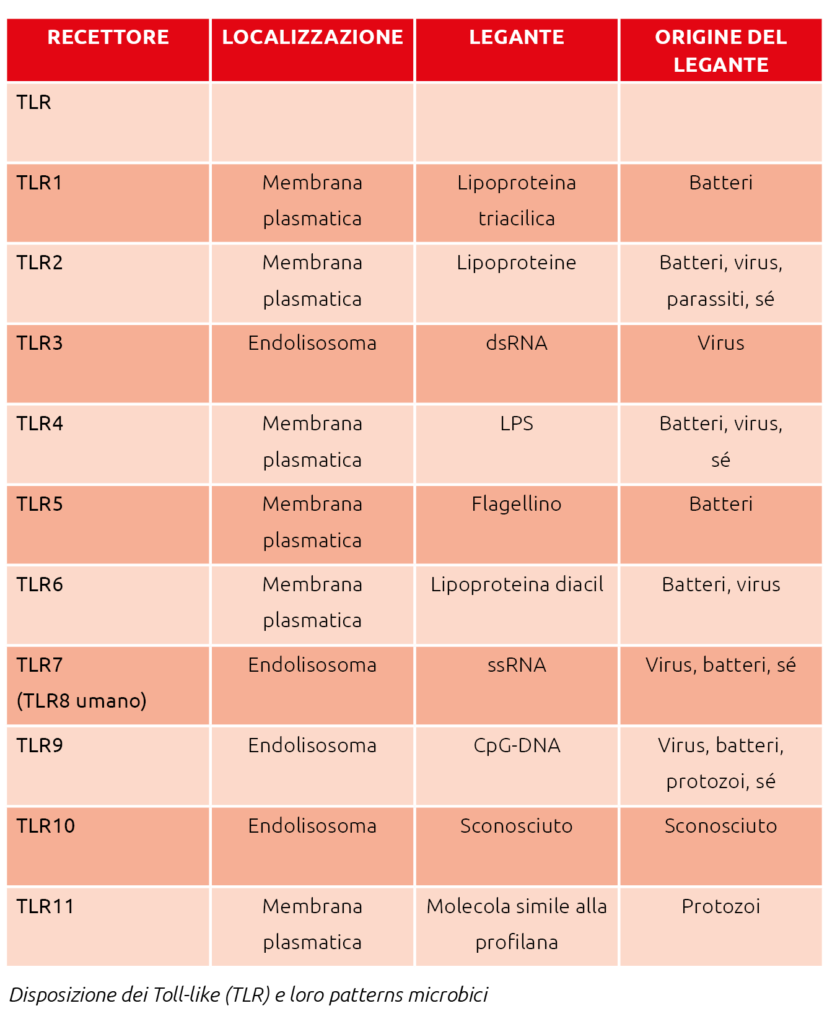
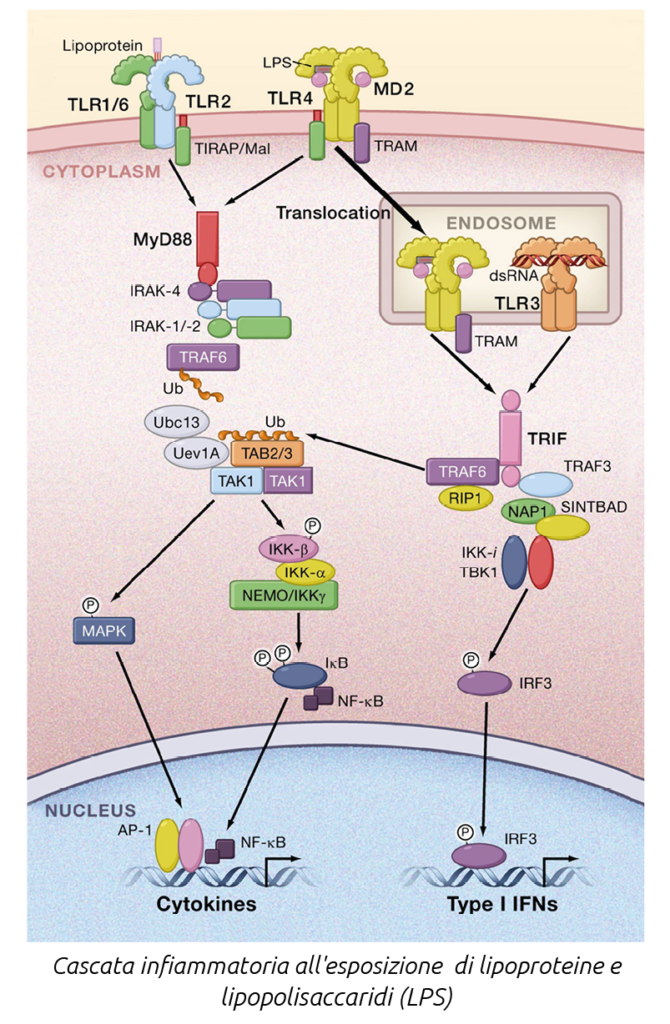
The initial perception of infection is mediated by recognition receptors that identify specific molecules, to which they respond by triggering a series of subsequent reactions.
There are several types of these receptors: Toll-like receptors (TLR), RIG-I-like receptors, NOD-like receptors, and C-type lectin receptors. Toll-like receptors (TLR) play a crucial role in host defense against microbial infections. However, the microbial patterns recognized by TLRs are not exclusive to pathogens but are also produced by commensals. It is believed that an inflammatory response to commensal bacteria is avoided through the recognition of specific molecules that detect microbial products, such as lipopolysaccharides (LPS) and lipoteichoic acid (LTA). TLRs function as sensors of microbial infection and are essential for initiating inflammatory responses and immune defense.
Experts recommend
Dietomilk
The milk replacer in cases of enteric issues

- Action on fecal consistency
- Nutritional
- Components with rehydrating action
Bireidral 1+2

Saline energetic rehydration for calves that supports a balanced start in fatigued and debilitated animals or as an adjunct in cases of digestive disorders.
To stay updated on all our news, follow us on Instagram or on Facebook.



